
Obtienen hidrógeno combustible a partir del agua del mar
La división del agua en hidrógeno y oxígeno presenta una alternativa a los combustibles fósiles, pero el agua es un recurso precioso. Un equipo de la Universidad de Stanford ha desarrollado una forma de aprovechar el agua de mar, a diferencia con los métodos que existían hasta la fecha, los cuales dependían de que el agua fuera muy pura.
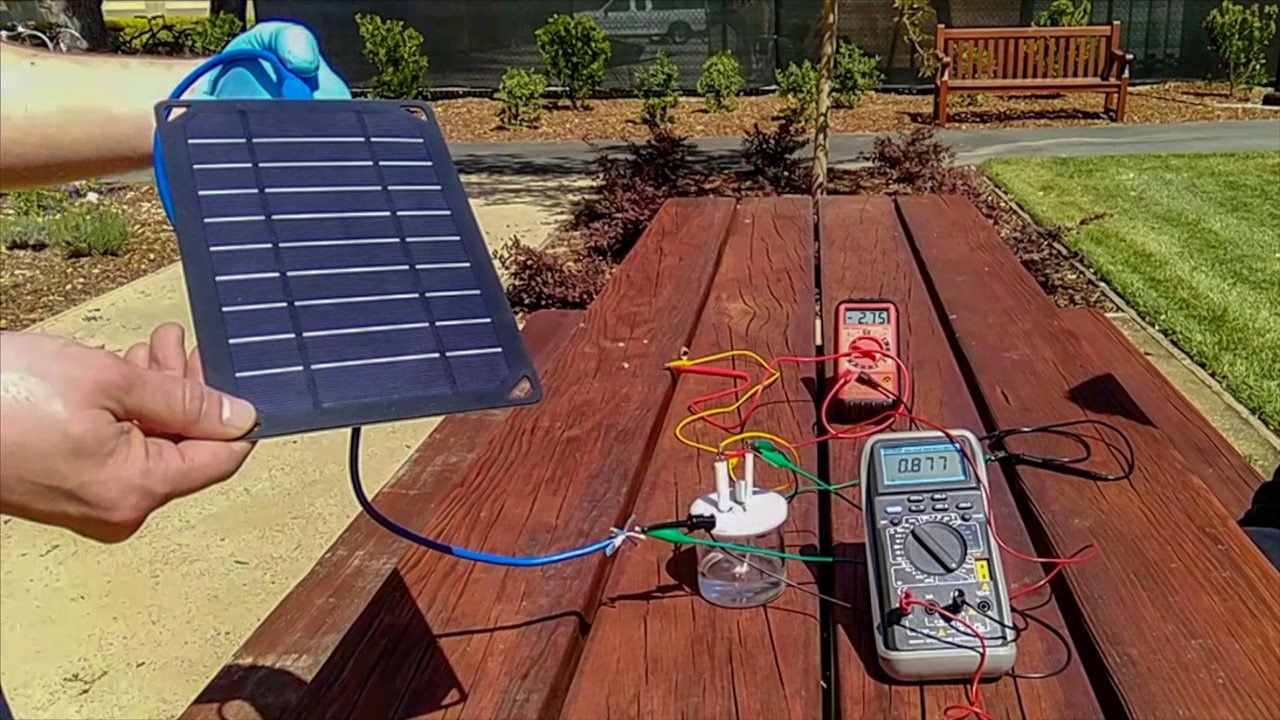
El hidrógeno combustible es una de las grandes apuestas para la obtención de energía en el futuro, pero hasta el momento el desarrollo de la tecnología necesaria para hacer este combustible una realidad contaba con un importante inconveniente: se necesitaba tanto hidrógeno como agua dulce hay en el mundo tan solo para cubrir las necesidades energéticas de las ciudades y el transporte de automóviles. No obstante todo parece haber mejorado gracias a los hallazgos de un equipo de investigadores de la Universidad de Stanford, quienes han ideado la manera de generar combustible de hidrógeno utilizando energía solar, electrodos y agua salada.
Los hallazgos, recogidos en el artículo “Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels” publicado recientemente en las actas de la Academia Nacional de Ciencias de Estados Unidos, han conseguido un nuevo sistema para separar el hidrógeno y el oxígeno del agua de mar a través de la electricidad. La diferencia con los métodos de separación de agua existentes hasta la fecha, es que estos dependían de que el agua fuera muy pura, lo cual es muy costoso de producir.

"El hidrógeno es una opción atractiva para ser utilizado como combustible porque no emite dióxido de carbono", declara Hongjie Dai químico y físico de la Universidad de Standford. La quema de hidrógeno produce solo agua como residuo y lo que debería ayudar con el problema de las emisiones de dióxido de carbono con respecto al cambio climático. El mismo Dai ha mostrado su cómo funciona su dispositivo en el laboratorio, pero ha declarado que cederá el diseño a los fabricantes a escala de este combustible para que puedan producirlo en masa.

El gran obstáculo: la corrosión
Como concepto, la división del agua en hidrógeno y oxígeno a partir de la electricidad, es una idea simple y antigua: es lo que los químicos y físicos denominan electrólisis. Para llevarla a cabo, una fuente de alimentación se conecta a dos electrodos colocados en el agua. Cuando se enciende la alimentación, salen burbujas de gas de hidrógeno del extremo negativo, llamado cátodo, y el oxígeno emerge en el extremo positivo, el ánodo.
No obstante el cloruro cargado negativamente presente en la sal de agua de mar puede corroer el extremo positivo, limitando la vida útil del sistema. Dai y su equipo querían encontrar una manera de evitar que los componentes del agua de mar rompieran los ánodos sumergidos y descubrieron que si cubrían el ánodo con capas ricas en cargas negativas, estas reducirían la descomposición del metal subyacente. Para ello usaron hidróxido de hierro y níquel y sulfuro de níquel, los cuales durante la electrólisis se convierten en una capa cargada negativamente que protege el ánodo.
"Sin el revestimiento con carga negativa, el ánodo solo funciona durante aproximadamente 12 horas en agua de mar", explica Michael Kenney, un estudiante graduado en el laboratorio de Dai y co-autor del estudio. "Todo el electrodo se desmorona", afirma Kenney. "Pero con esta capa, es capaz de funcionar durante más de mil horas".
Los estudios anteriores que intentaron dividir el agua de mar para el combustible de hidrógeno habían funcionado con cantidades bajas de corriente eléctrica, porque la corrosión se produce en corrientes más altas. Pero Dai, Kenney y sus colegas pudieron conducir hasta 10 veces más electricidad a través de su dispositivo de múltiples capas, lo que ayuda a generar hidrógeno a partir del agua de mar a una velocidad mayor. "Creo que establecimos un récord en la corriente para dividir el agua de mar", declara Dai. "Pero lo impresionante de este estudio fue que pudimos operar con corrientes eléctricas que son las mismas que se usan en la industria hoy en día", añade Kenney.
Ahora que la receta básica está diseñada para la electrólisis con agua de mar, este nuevo método abrirá las puertas para aumentar la disponibilidad de combustible de hidrógeno alimentado por energía solar o eólica. En el futuro, la tecnología podría utilizarse para fines más allá de la generación de energía. Dado que el proceso también produce oxígeno respirable, los buzos o submarinos podrían llevar sus dispositivos al océano y generar oxígeno sin tener que salir a la superficie para respirar aire.




